LDGraft™, is our 100% pre-encapsulated rhBMP-2 product.:
Bone healing is a carefully orchestrated biological process, moving through distinct phases—from inflammation to repair and, ultimately, remodeling [1]. While recombinant human Bone Morphogenetic Protein-2 (rhBMP-2) has long been recognized for its osteoinductive potential, how and when it’s delivered to the site of injury can significantly impact the healing response [2].
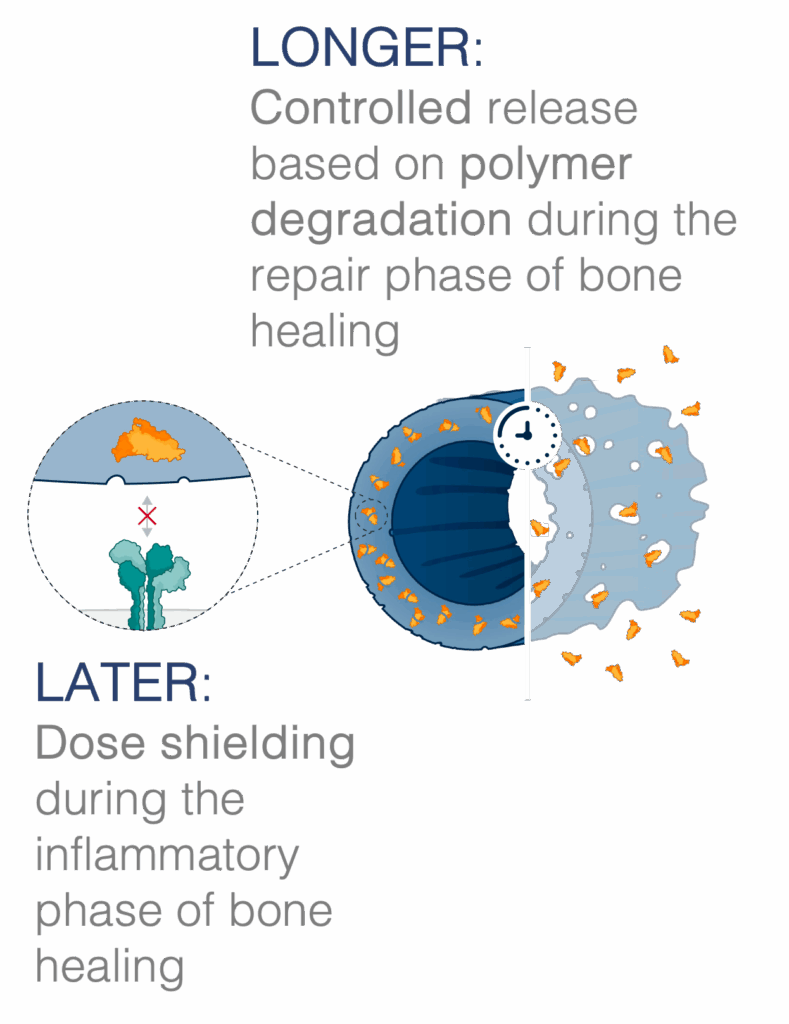
Surface-bound rhBMP-2 may present too much, too soon.
When rhBMP-2 is exposed in large amounts at the surface of a device or graft, it delivers a supraphysiological signal during the inflammatory phase of bone healing [3]. rhBMP-2 has been shown in preclinical studies to produce an exaggerated inflammatory response [4-6]. This “too much, too soon” of surface attached rhBMP-2 delivery produces a compounding effect during a period of peak inflammation in bone healing.
BMP-2 naturally peaks during the repair phase of bone healing. There is a clear delay in BMP-2 expression in human bone healing, and it does not reach a peak until 7-14 days7. Mimicking this delayed presentation has consistently been shown to lead to improved bone healing [2,8].
LDGraft is designed with this timing in mind. By encapsulating the rhBMP-2, LDGraft uniquely provides dose shielding during the early inflammatory phase and enables an extended release over 4-6 weeks [9,10]. LDGraft’s design intent is to shift the majority of rhBMP-2 release to the repair phase—better aligned to the body’s natural signaling timeline.
What is the preclinical evidence?
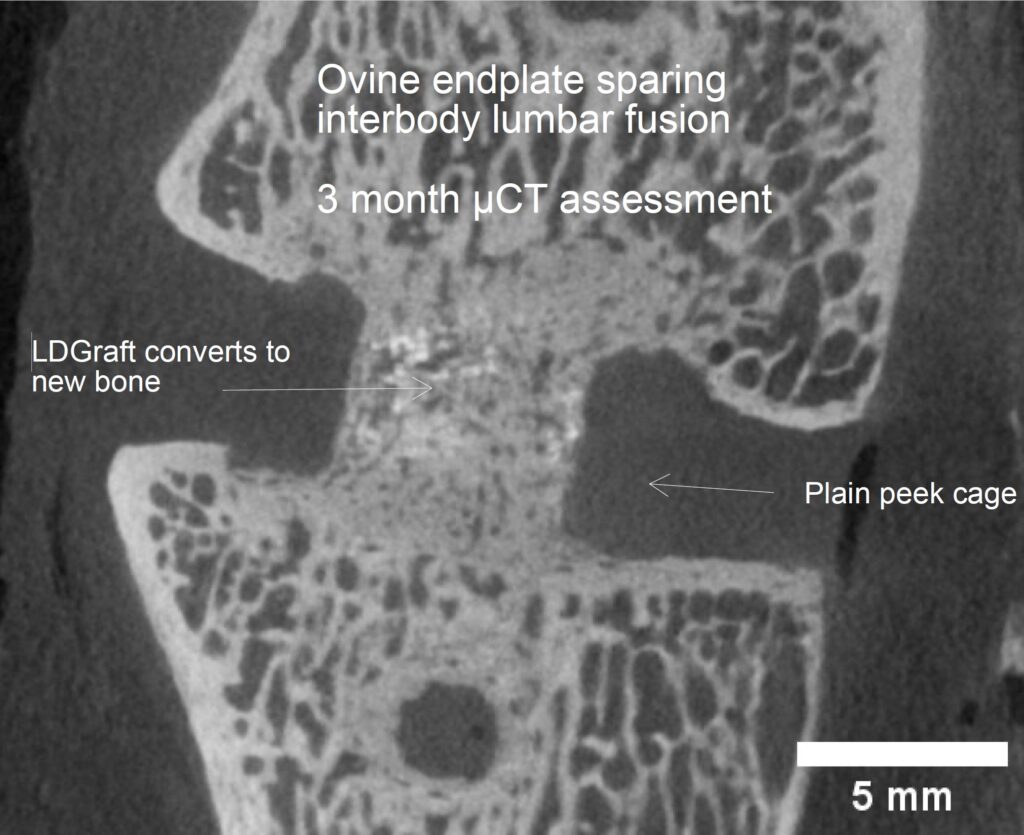
We have completed an endplate sparing lateral interbody fusion model in 120 sheep. Our results have been presented at both Spine Week (2023) and NASS (2023).
We reported all three rhBMP-2 doses tested were equivalent to iliac crest bone graft in terms of fusion at 6 months. Importantly, our controlled and extended-release product showed no signs of osteolysis or ectopic bone growth6,7.
** Note: Preclinical results may not be representative of clinical outcomes.
LDGraft has also received an FDA Breakthrough Device designation for the Anterior Lumbar Interbody Fusion (ALIF) indication at one level from L3-S1 for skeletally mature patients with degenerative disc disease.

*For investigational use only, not approved for sale
References:
- Loi, F., et al., Inflammation, fracture and bone repair. Bone, 2016. 86: p. 119-30.
- Seeherman, H., et al., rhBMP-2/calcium phosphate matrix accelerates osteotomy-site healing in a nonhuman primate model at multiple treatment times and concentrations. J Bone Joint Surg Am, 2006. 88(1): p. 144-60.
- Seeherman, H., et al., Injectable rhBMP-2/CPM paste for closed fracture and minimally invasive orthopaedic repairs. J Musculoskelet Neuronal Interact, 2003. 3(4): p. 317-9; discussion 320-1.
- Liau, Z.Q.G., et al., Dose-dependent Nerve Inflammatory Response to rhBMP-2 in a Rodent Spinal Nerve Model. Spine, 2017. 42(16).
- Xiong, C., et al., BMP-2 adverse reactions treated with human dose equivalent dexamethasone in a rodent model of soft-tissue inflammation. Spine (Phila Pa 1976), 2013. 38(19): p. 1640-7.
- Wang, M., et al., Minimizing the severity of rhBMP-2-induced inflammation and heterotopic ossification with a polyelectrolyte carrier incorporating heparin on microbead templates. Spine (Phila Pa 1976), 2013. 38(17): p. 1452-8.
- Von Benecke, J., et al., A Narrative Review on Recombinant Human Bone Morphogenetic Protein 2: Where Are We Now? Cureus, 2024. 16.
- Virk, S., et al., Pilot Study on Percutaneous Delivery of Recombinant Human Bone Morphongenetic Protein-2 Augments Fusion in a Nicotine-impaired Rabbit Fusion Model. Clin Spine Surg, 2023. 36(10): p. E512-e518.
- LocateBio, Data on file – LBI2021-1.
- LocateBio, Data on file – LDG-R-032.